NS96 DISC REMOVER
MPIN: MP41169
Sign in to view priceReliable Newborn Screening devices provided by Labsystems Diagnostics.
Ask for Quote
Reliable Newborn Screening devices provided by Labsystems Diagnostics.
OVERVIEW
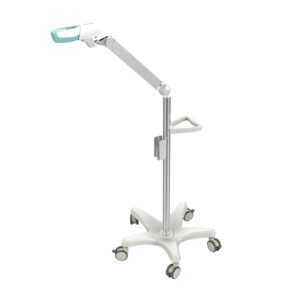
Stand-alone NS96 Disc Remover effectively removes the sample discs after incubation and reduces the chances of cross-contamination
Shipping Policy
Orders made at Medpick are initiated and processed for shipment upon receipt of request from the customer. Please note that our Shipping Services (Fee, Transportation, Loss or Damage of any shipment, etc.) are in accordance with the Seller\'s terms of Shipment.
Refund Policy
Please refer to Medpick Return Policy.
Cancellation / Return / Exchange Policy
Please refer to Medpick Return Policy.
 REGISTER
REGISTER
 SIGN IN
SIGN IN