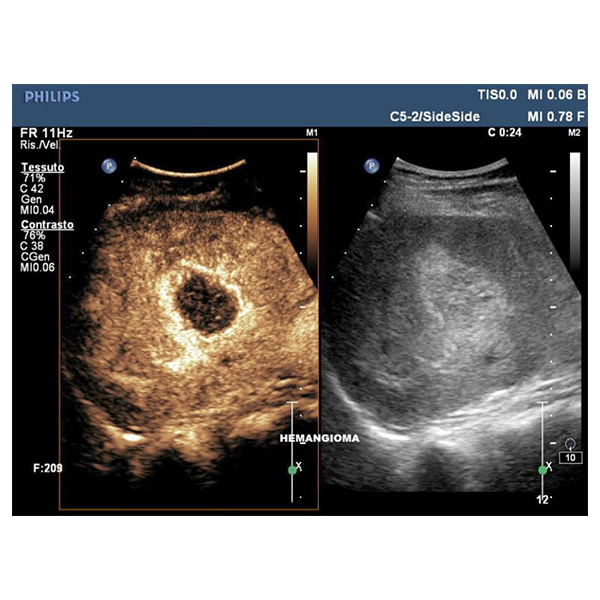
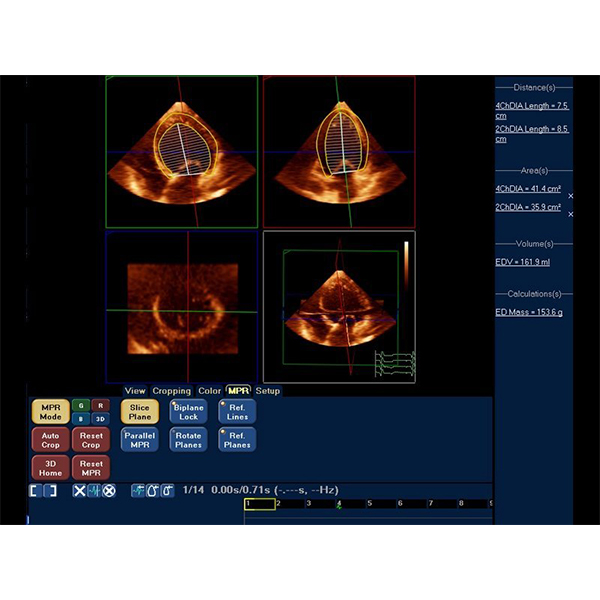
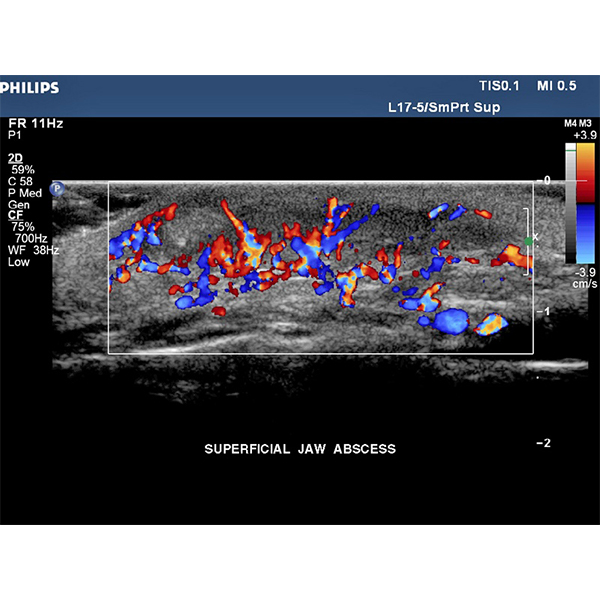
Philips iU22 Ultrasound Machine –
MPIN: MP11720
Sign in to view priceAsk for Quote
Overview
Application training for the Philips iU22
KPI’s on-staff sonographer can provide onsite applications training or remote training via videoconference at a set price plus travel costs. A pre-recorded video training course is included in the sale, lease or rental of the Philips iU22 from KPI Healthcare.
Philips iU22 Service options
Free technical support is available from KPI during installation and over the course of the standard limited warranty. Technical support is available after the warranty period at an hourly cost per issue.
Philips iU22 Maintenance
KPI recommends the use of a surge protector along with a dedicated power outlet. Probes should be disinfected after every use with a disinfectant wipe proven not to damage the lens (KPI recommends SonoWipes for this.) KPI recommends one PM visit (preventative maintenance) every year.
Philips iU22 Dimensions & Weight
Height: (adjustable, maximum) 1626 mm (64 in), (minimum) 1397 mm (55 in)
Width: 559 mm (22.0 in)
Depth: 1092 mm (43 in)
Weight: (no Peripherals) 156.8 kg (345 lbs.), approx. 480 lbs with packaging
iU22 Specifications
Philips xSTREAM imageformer architecture
Up to 442,176 total digital channels (xMATRIX configuration), 57,000 total digital channels (non xMATRIX configuration)
Displayed Imaging Depth: 1 – 39 cm (transducer dependent)
Up to 180 dB system dynamic range
Philips iU22 Electrical power
Voltage 100V-127V, 220V-240V
Frequency 50/60 Hz
Power consumption: 750VA – 900VA depending on system configuration
Revisions
Philips iU22 Revisions: A cart – C cart
Philips first launched the iU22 in 2004 as their new premium shared service ultrasound machine, replacing older units like the HDI 5000. This first version of hardware was designated an “A-cart.” This first version had dual motherboards and dual power supplies and a huge amount of problems with both. The iU22 B-cart improvements were to circuit boards, cables, power supply assembly, and USB ports. The iU222 C-cart brought a new 20” LCD monitor and articulating arm as well as new circuit boards. The R-cart is a A,B, or C-cart with hardware and software upgrades to G4.0 software.
Philips iU22: D cart – F cart
The iU22 D-cart was launched in 2007 and finally fixed the problems with the dual motherboards by using a unified motherboard. D.1 and D.2 included minor hardware updates. The iU22 E-cart made upgrades to the cart, wheels and locking mechanism. E.1 was a minor hardware & software update. E.2 added major power supply improvements to support Matrix transducers. E.3 was a minor update to the way the iU22 was manufactured. The iU22 F-cart was a major upgrade that added Type III channel boards. F.1 was a minor manufacturing update. F.2 upgraded the video card. F.3 upgraded to a new SATA DVD drive.
Philips iU22: G cart
The iU22 G-cart was launched in 2010 and is the latest version of the iU22. This revision added a new channel board and a 21” widescreen monitor. The iU22 G.1 revised the control panel and added larger touch screens. KPI recommends buying D-cart and up as most hardware problems with the iU22 were fixed by this revision and D-carts can be updated to the latest software
Philips iU22 Vision software updates
The software of an iU22 can be updated to a point limited by the hardware cart. Each major revision of the software was designated with the year and “Vision” in the name. “Vision 2012” was the last major release of the software. Very early A-C cart had software that was named simply “system update” with a number. System update 1.0-2.0 have almost all been upgraded over the years and system 3.0 is the lowest version still commonly found on used iU22s.
The Philips iU22 Vision 2007 software update included support for the new L15-7io intraoperative linear transducer and the V6-2 4D convex probe. Features added were Qlab’s iSlice, STIC, TCD (transcranial Doppler) imaging, TCD TSI, and Color compare imaging. Improvements were made to workflow, and image quality on a number of existing transducers.
The Philips iU22 Vision 2008 software update included support the new Purewave C5-1 convex transducer. Features added were TMQ (tissue motion quantification) in Qlab, Viewforum stand alone image viewing platform, improved needle visualization, and contrast capabilities.
The Philips iU22 Vision 2009 software update included support for the new VL13-5 4D linear probe. Features added were tissue aberration correction, contrast enhanced ultrasound, and smartexam automation software.
The Vision 2010 software update on the Philips iU22 added xMatrix including support for the new Purewave xMatrix X6-1 sector transducer, Live xPlane imaging, and support for any PACS. (R-carts can have this update, but cannot support the X6-1 transducer.)
The Philips iU22 Vision 2011 software update added support for the X5-1 xMatrix sector transducer. Qlab was upgraded with CMQ-Stress replacing TMQ, and with the additions of the Auto Volume tool, GI 3DQ trending, iCrop, and Elevation compounding. Options with Vision 2011 include the xMatrix performance bundle, and Live 3D. (R-carts can have this update, but cannot support the X5-1 transducer.)
The Vision 2012 software on the Philips iU22 adds support for Auto Doppler, Fetal Heart Navigator, and Vascular Plaque Quantification (VPQ), and GYN Elastography.
All revisions of the Philips iU22:
Philips iU22 (A.0) with system update 3.0 – Vision 2010 software
Philips iU22 (B.0) with system update 3.0 – Vision 2010 software
Philips iU22 (C.0) with system update 3.0 – Vision 2010 software
Philips iU22 (R.0) with Vision 2007-Vision 2011 software (limited)
Philips iU22 (D.0) with Vision 2007 – Vision 2012 software
Philips iU22 (D.1) with Vision 2007 – Vision 2012 software
Philips iU22 (D.2) with Vision 2007 – Vision 2012 software
Philips iU22 (E.0) with Vision 2007 – Vision 2012 software
Philips iU22 (E.1) with Vision 2007 – Vision 2012 software
Philips iU22 (E.2) with Vision 2007 – Vision 2012 software
Philips iU22 (E.3) with Vision 2007 – Vision 2012 software
Philips iU22 (F.0) with Vision 2007 – Vision 2012 software
Philips iU22 (F.1) with Vision 2007 – Vision 2012 software
Philips iU22 (F.2) with Vision 2007 – Vision 2012 software
Philips iU22 (F.3) with Vision 2007 – Vision 2012 software
Philips iU22 (G.0) with Vision 2011 or Vision 2012 software
Philips iU22 (G.1) with Vision 2011 or Vision 2012 software
Probes
All Philips iU22 Probes / Transducers
4D Convex: V6-2 [ 2 – 6 MHz ] 192 elements, 55mmR, 100° x 85° volume field of view
4D Endocavitary: 3D9-3v [ 3 – 9 MHz ] 128 elements, 26.1mm, 156° x 85° volume field of view
4D Linear: VL13-5 [ 5 – 13 MHz ] 192 elements, 38.4mm, 38 mm x 30° volume field of view
Linear L9-3: [ 3 – 9 MHz ] 160 elements, 38mmR
Linear L12-5 50 mm: [ 5 – 12 MHz ] 256 elements, 50mmR
Linear: L17-5 [ 5 – 18 MHz ] 288 elements, 38.9mm, ultra-fine pitch
Intraoperative Linear: L15-7io [ 7 – 15 MHz ] 128 elements, 23mm,
Convex C5-1: [ 1 – 5 MHz ] 160 elements, 96° field of view, PureWave Crystal Technology
Convex C5-2:[ 2 – 5 MHz ] 128 elements, 115° field of view
Microconvex C8-5: [ 5 – 8 MHz ] 128 elements, 120° field of view, Pediatric imaging
Convex C9-4 :[ 4 – 9 MHz ] 192 elements, 108° field of view
Endovaginal C8-4v: [ 4 – 8 MHz ] End-fire sector, 128 elements, 11mmR, 160° field of view
Endocavitary C9-5ec: [ 5 – 9 MHz ] End-fire sector, 128 elements, 8mmR, 173° field of view
Endovaginal C10-3v: [ 3 – 10 MHz ] End-fire sector, 128 elements, 11.5mmR, 164° field of view
Cardiac Sector:S3-1 [ 1 – 3 MHz ] 96 elements, 90° field of view, adult cardiac sector
Cardiac Sector : S4-1 [ 1 – 4 MHz ] 96 elements, 90° field of view, adult cardiac sector
Cardiac Sector : S5-1 [ 1 – 5 MHz ] 80 elements, 90° field of view, PureWave crystal technology
TEE S7-2omni: [ 2 – 7 MHz ] 64 elements, 90° field of view, Transesophageal TEE
TEE S7-2:[ 2 – 7 MHz ] 64 elements, 90° field of view, Transesophageal TEE
xMATRIX: X3-1 [ 1 – 3 MHz ] 2,400 element/.s, 90° field of view
xMATRIX: X5-1 [ 1 – 5 MHz ] 3,040 elements, 100° field of view, PureWave crystal technology
xMATRIX: X6-1 [ 1 – 6 MHz ] 9,212 elements, 100° field of view, PureWave crystal technology
xMATRIX:X7-2 [ 2 – 7 MHz ] 2,500 elements, 90° field of view, PureWave crystal technology
xMATRIX TEE transesophegeal: X7-2t [ 2 – 7 MHz ] 2,500 elements, 90° field of view, PureWave crystal technology
Pedoff (CW Transducer): D2TCC [ 2 MHz ] Transcranial Doppler applications
Pedoff (CW Transducer): D2CWC [ 2 MHz ] Adult cardiology applications
Pedoff (PW Transducer): D5CWC [ 5 MHz ] Deep venous and arterial applications
Advanced Philips iU22 Transducers: 4D and Matrix
The Philips iU22 not only offers six different PureWave single crystal transducers, but five xMatrix probes. xMatrix not only improves image quality and penetration like a PureWave transucer, but also allows for real-time scanning of multiple planes and 4D without needing a typical mechanical 4D probe. This speeds the acquisition of 4D data over a 4D mechanical probe. C-plane resolution is also improved over a typical 4D mechanical probe. The [ 1 – 6 MHz ] X6-1, and [ 1 – 5 MHz ] X5-1 transducers for the iU22 represent the apex of xMatrix design and can image anything from the heart, to a 4D OB/GYN exam. The [ 1 – 3 MHz ] X3-1 and [ 2 – 7 MHz ] X7-2 are early xMatrix probes, while the [ 2 – 7 MHz ] X7-2t is a TEE probe with xMatrix technology and represents the pinnacle of transesephegeal technology. In addition, the iU22 has 3 traditional 4D transducers including the [ 5 – 13 MHz ] VL13-5 4D linear.
Popular Philips iU22 Transducers
The most popular transducers for the iU22 are the [ 1 – 5 MHz ] C5-1 PureWave convex which greatly enhances image quality at higher penetration where patients are heavier and would otherwise be difficult to image. The [ 3 – 10 MHz ] C10-3v PureWave endovaginal which also uses single crystal PureWave technology to improve image quality with difficult-to-image patients. The most popular linear for the Philips iU22 is the [ 5 – 12 MHz ] L12-5 50 mm offers excellent vascular imaging as well as having a large enough scan head for effecient breast imaging. The most popular cardiac sector transducer is the [ 1 – 5 MHz ] S5-1 another PureWave transducer offering better than normal image clarity even at deep penetration. The most popular 4D mechanical transducers on the Philips iU22 are the [ 2 – 6 MHz ] V6-2 4D convex, and the [ 3 – 9 MHz ] 3D9-3v 4D endovaginal transducer. 4D probes are superior to 2D transducers because they capture an entire exam’s worth of data in a few seconds of scanning rather than many minutes. There are also views of the internal structures, such as the coronary view or C-plane of the uterus that are only viewable in 3D/4D on an ultrasound and cannot be imaged at all with a 2D version of the probe.
Competitors
How the Philips iU22 compares with other Philips systems
The Siemens Philips iU22 was launched in 2004 as the replacement to the Philips HDI 5000 and continued in production until 2015. The iU22 is a premium level ultrasound machine as was top of the line till the introduction of the Epiq line in 2013. The Epiq 5 replaced the iU22 and both are both use single crystal transducers, but the Epiq 5 does not have xMatrix probes. The Epiq 5 does have a larger monitor and touchscreen, and is sized smaller than the iU22. The HD15 sits below the iU22 in features, image quality and price.
Philips iU22 vs iE33
When Philips launched the iU22 it launched the iE33 at the same time and ever since, many medical professionals have wondered what the difference is, and which is best for their needs. The iE33 is focused only on cardio vascular applications where the iU22 is shared service and focused on women’s health, general imaging, and cardiac applications. The iE33 and iU22 even look nearly identical; with the major distinguishing feature being dual touchscreens for navigation on the iE33 while the iU22 has only one touchscreen.
Other brands competing with the Philips iU22
The Siemens Philips iU22 is a true shared-service ultrasound machine able to do all applications from 4D OB/GYN to full cardiac exams. Its closest competitor from GE would be the Logiq E9 and both systems are very similar though the Logiq E9 has superior 4D imaging for OB/GYN while the iU22 offers superior cardiac including 4D cardiac and a pediatric TEE probe. The closest competitor from Samsung is the WS80A which again offers 4D for OB/GYN applications superior to the iU22 but has only some pediatric cardiac support.
Features
Tissue Harmonic Imaging : Yes
Spatial Compounding(=CrossXbeam) : Yes
Speckle Reduction (=SRI) : Yes
Auto Image Opt(B mode) : Yes
Auto Image Opt(Doppler): Yes
Write Zoom: Yes
Triplex Mode: Yes
Needle Enhancement or Needle Recognition :Yes
Auto NT Measurement (=Sono NT)
:No
Auto Follicle 2D Measurement:No
Auto Follicle 3D Measurement:No
Auto IMT:Yes
Auto IMT (Real Time):No
Automated B/M/D Measurement:Yes
Automated LH Measurement(Automated Function Imaging(AFI), Cardiac Motion Quantification(CMQ), or Auto EF(Ejection Fraction):Yes
Live Dual (B/BC) Mode:Yes
SmartExam or Scan Assistant:Yes
Independent Steer & Lockable Wheels:Yes
Fusion:Yes
Raw Data File:Yes
Flexible Report:Yes
Barcode Reader:No
Gel Warmer:No
Transducers
Convex (1~6Mhz):Yes
Convex (2~9Mhz):Yes
Single Crystal Convex (1~6Mhz):Yes(1~5Mhz)
Single Crystal Convex (2~9Mhz):No
2D Arrary 3D Convex (1~6Mhz):Yes
Micro Convex (5~8Mhz):Yes
Single Crystal Endocavity_Straight Type (3~10Mhz):Yes(3~9Mhz)
Endocavity_Curved Type (5~8Mhz):No
3D Convex (2~6Mhz):No
3D Convex Light Weight (2~7Mhz):No
3D Endocavity (3~10Mhz):Yes
3D Micro Convex (3~9Mhz):No
3D Linear (4~18Mhz):No
Linear (>14Mhz):No
Linear (3~12Mhz):Yes
Linear (<9Mhz):Yes
Single Crystal Linear (>14Mhz):No
Single Crystal Linear (3~12Mhz):No
Single Crystal Linear (<9Mhz):No
Linear 50mm:Yes
Linear 25mm:No
Hockey stick (<13Mhz):Yes
Hockey stick (>13Mhz):Yes(7~15Mhz io)
T or L shape Intra Operative:Yes
Phased Array_Adult (1~5Mhz):Yes
Single Crystal Phased Array_Adult (1~5Mhz):Yes
2D Arrary 3D Phased Array (1~5Mhz):No
Phased Array_Pediatric (3~8hz):Yes
Single Crystal Phased Array_Pediatric (3~8hz):No
Phased Array_Neonate (4~12Mhz):
ICE (Intracardiac Echo Cardiography):No
TEE_Adult (3-7Mhz):Yes
TEE_Pediatric (3~7Mhz):No
2D Array 3D TEE (2~7Mhz):Yes
Pencil CW (2Mhz):Yes
Pencil CW (5 or 6Mhz):Yes
Imaging Modes
2D, M mode:Yes
M-color Flow Mode:Yes
Anatomical M-mode:Yes
Trapezoidal Mode:Yes
Color, Power Angio, Pulse Wave Doppler:Yes
Bi-directional Power (=HD FLOW):Yes
SCW Doppler:Yes
Tissue Doppler(Velocity) Imaging:Yes
Freehand 3D:Yes
Live 3/4D OB/GYN:No
HD Live:No
STIC (Spatio-Temporal Image Correlation):No
Live 3D Echo:No
Stress Echo:No
Strain and Strain Rate (Cardiac):Yes
B Flow:No
Panoramic Imaging (=Logiq view):No
Contrast Imaging – Cardiac:Yes
Contrast Imaging – General Imaging:Yes
Strain-based Elastography:No
Shear Wave Elastography:No
Applications
Abdominal:Yes
Women’s Health Care (GYN & Breast):Yes
OB:Yes
Fetal Echo:Yes
Vascular:Yes
TCD(Transcranial):Yes
Small Parts (Breast, Thyroid, Testis…):Yes
MSK/Anesthesiology:Yes
Pediatrics:Yes
Urology (Renal, Prostate…):Yes
Echocardiography_Adult:Yes
Interventional Cardiology:Yes
Echocardiography_Pediatric:No
Echocardiography_Neonate:Yes
Stress Echocardiography:Yes
Transesophageal Echo_Adult:Yes
Transesophageal Echo_Pediatric:No
Internal Medicine w/ Shared Service:Yes
Surgury:No
Interventional Radiology:Yes
Contrast Imaging _ General Imaging (Low MI):No
Contrast Imaging _ Cardiac (High or Low MI): Yes
Bowel Imaging:No
Strain Elastography:No
Shear Wave Elastography:No
Specification
System Overview
Year Launched: 2004
Estimated Market Price ($): Premium
Monitor (inch): 17″LCD(20″ option)
Tilt/Rotate Adjustable Monitor: Yes
Monitor Resolution:
Image Size Resolution:
Touch Screen (Inch): Yes
Trackball or Trackpad: Trackball
CP Back-Lighting:Yes
Weight:345lbs
Probe Ports: 3
Battery:No
Boot-Up Time:120sec
Sleep Mode (Quick Start):30sec(stanby mode)
Maximum Depth of Field:35cm
Minimum Depth of Field:2cm
Cart (HCU):No
Independent Steer & Lockable Wheels:Yes
Connectivity
DICOM 3.0:Yes(Option)
DICOM SR_Cardiac:Yes
DICOM SR_Vascular :Yes
DICOM SR_OB/GYN:Yes
JPEG, WMV, & AVI:Yes
USB:Yes
HDD/SDD:160GB
DVD/CD RW:Yes
Wireless LAN:No
Shipping Policy
Orders made at Medpick are initiated and processed for shipment upon receipt of request from the customer. Please note that our Shipping Services (Fee, Transportation, Loss or Damage of any shipment, etc.) are in accordance with the Seller\'s terms of Shipment.
Refund Policy
Please refer to Medpick Return Policy.
Cancellation / Return / Exchange Policy
Please refer to Medpick Return Policy.
 REGISTER
REGISTER
 SIGN IN
SIGN IN